Leading Manufacturer for Latuda Lurasidone - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN
Leading Manufacturer for Latuda Lurasidone - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN
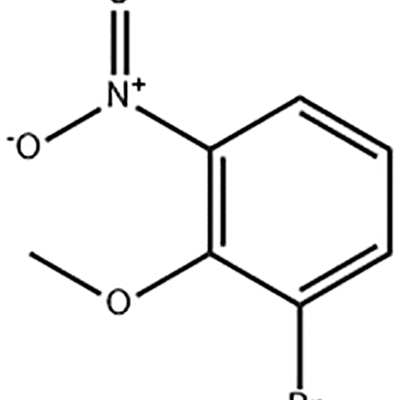
Leading Manufacturer for Latuda Lurasidone - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN Detail:
1-Bromo-2-methoxy-3-nitro-benzene is used as the intermediate of Eltrombopag .
Eltrombopag, developed by GlaxoSmithKline (GSK) in the UK and later jointly developed with Novartis in Switzerland, is the first and only approved small molecule non peptide TPO receptor agonist in the world. Eltrombopag was approved by the US FDA in 2008 for the treatment of idiopathic thrombocytopenic purpura (ITP), and in 2014 for the treatment of severe aplastic anemia (AA). It is also the first drug approved by the US FDA for the treatment of AA in recent 30 years.
In december2012, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in patients with chronic hepatitis C (CHC), so that hepatitis C patients with poor prognosis due to low platelet count can start and maintain interferon based standard therapy for liver diseases. On february3,2014, GlaxoSmithKline announced that the FDA granted the breakthrough treatment drug qualification of Eltrombopag for the treatment of hemopenia in patients with severe chemicalbook aplastic anemia (SAA) who did not fully respond to immunotherapy. On August 24, 2015, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in adults and children aged 1 year and over with chronic immune thrombocytopenia (ITP) who have insufficient response to corticosteroids, immunoglobulins or splenectomy. On january4,2018, Eltrombopag was approved to be listed in China for the treatment of primary immune thrombocytopenia (ITP).
Product detail pictures:
Related Product Guide:
Just about every member from our large efficiency income crew values customers' wants and enterprise communication for Leading Manufacturer for Latuda Lurasidone - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN , The product will supply to all over the world, such as: Swedish, Haiti, Plymouth, We adhere to the honest, efficient, practical win-win running mission and people-oriented business philosophy. Excellent quality, reasonable price and customer satisfaction are always pursued! If you are interested in our items, just try to contact us for more details!
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!


![factory customized Membrane Water Treatment - 1,4′-Bipiperidine]-1′-carbonyl chloride HCl – JIN DUN](https://cdn.globalso.com/jindunchem-med/21.png)
![China New Product Sertraline Antipsychotic - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)