Low MOQ for Ciprofloxacin Hcl 93107-08-5 - 2′-Methoxy-3′-nitro-biphenyl-3-carboxylic acid – JIN DUN
Low MOQ for Ciprofloxacin Hcl 93107-08-5 - 2′-Methoxy-3′-nitro-biphenyl-3-carboxylic acid – JIN DUN
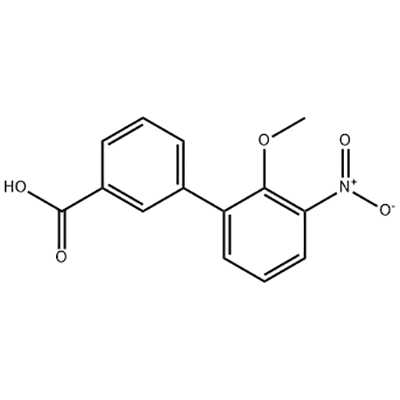
Low MOQ for Ciprofloxacin Hcl 93107-08-5 - 2′-Methoxy-3′-nitro-biphenyl-3-carboxylic acid – JIN DUN Detail:
2′-Methoxy-3′-nitro-biphenyl-3-carboxylic acid is used as the intermediate of Eltrombopag .
Eltrombopag, developed by GlaxoSmithKline (GSK) in the UK and later jointly developed with Novartis in Switzerland, is the first and only approved small molecule non peptide TPO receptor agonist in the world. Eltrombopag was approved by the US FDA in 2008 for the treatment of idiopathic thrombocytopenic purpura (ITP), and in 2014 for the treatment of severe aplastic anemia (AA). It is also the first drug approved by the US FDA for the treatment of AA in recent 30 years.
In december2012, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in patients with chronic hepatitis C (CHC), so that hepatitis C patients with poor prognosis due to low platelet count can start and maintain interferon based standard therapy for liver diseases. On february3,2014, GlaxoSmithKline announced that the FDA granted the breakthrough treatment drug qualification of Eltrombopag for the treatment of hemopenia in patients with severe chemicalbook aplastic anemia (SAA) who did not fully respond to immunotherapy. On August 24, 2015, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in adults and children aged 1 year and over with chronic immune thrombocytopenia (ITP) who have insufficient response to corticosteroids, immunoglobulins or splenectomy. On january4,2018, Eltrombopag was approved to be listed in China for the treatment of primary immune thrombocytopenia (ITP).
Product detail pictures:
Related Product Guide:
Being supported by an innovative and experienced IT team, we could present technical support on pre-sales & after-sales service for Low MOQ for Ciprofloxacin Hcl 93107-08-5 - 2′-Methoxy-3′-nitro-biphenyl-3-carboxylic acid – JIN DUN , The product will supply to all over the world, such as: Grenada, Argentina, Philadelphia, High output volume, top quality, timely delivery and your satisfaction are guaranteed. We welcome all inquiries and comments. We also offer agency service---that act as the agent in china for our customers. If you are interested in any of our products or have an OEM order to fulfill, please feel free to contact us now. Working with us will save you money and time.
Adhering to the business principle of mutual benefits, we have a happy and successful transaction, we think we will be the best business partner.