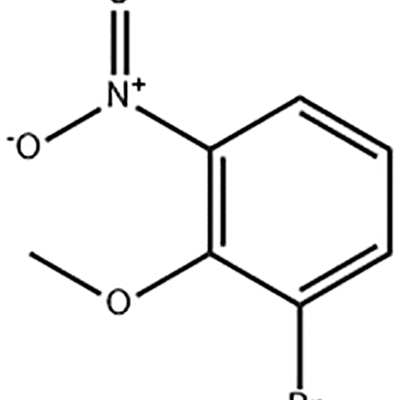
Original Factory Vascular Physiology - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN
Original Factory Vascular Physiology - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN
Original Factory Vascular Physiology - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN Detail:
1-Bromo-2-methoxy-3-nitro-benzene is used as the intermediate of Eltrombopag .
Eltrombopag, developed by GlaxoSmithKline (GSK) in the UK and later jointly developed with Novartis in Switzerland, is the first and only approved small molecule non peptide TPO receptor agonist in the world. Eltrombopag was approved by the US FDA in 2008 for the treatment of idiopathic thrombocytopenic purpura (ITP), and in 2014 for the treatment of severe aplastic anemia (AA). It is also the first drug approved by the US FDA for the treatment of AA in recent 30 years.
In december2012, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in patients with chronic hepatitis C (CHC), so that hepatitis C patients with poor prognosis due to low platelet count can start and maintain interferon based standard therapy for liver diseases. On february3,2014, GlaxoSmithKline announced that the FDA granted the breakthrough treatment drug qualification of Eltrombopag for the treatment of hemopenia in patients with severe chemicalbook aplastic anemia (SAA) who did not fully respond to immunotherapy. On August 24, 2015, the US FDA approved Eltrombopag for the treatment of thrombocytopenia in adults and children aged 1 year and over with chronic immune thrombocytopenia (ITP) who have insufficient response to corticosteroids, immunoglobulins or splenectomy. On january4,2018, Eltrombopag was approved to be listed in China for the treatment of primary immune thrombocytopenia (ITP).
Product detail pictures:
Related Product Guide:
High quality Very first,and Consumer Supreme is our guideline to offer the most beneficial service to our consumers.At present, we're attempting our greatest to be among the top exporters in our area to fulfill buyers far more need to have for Original Factory Vascular Physiology - 1-Bromo-2-methoxy-3-nitro-benzene – JIN DUN , The product will supply to all over the world, such as: Jamaica, Puerto Rico, Rome, As the world economic integration bringing challenges and opportunities to the xxx industry, our company , by carrying on our teamwork, quality first, innovation and mutual benefit, are confident enough to provide our clients sincerely with qualified products, competitive price and great service, and to build a brighter future under the spirit of higher, faster, stronger with our friends together by carrying on our discipline.
Perfect services, quality products and competitive prices, we have work many times, every time is delighted, wish continue to maintain!