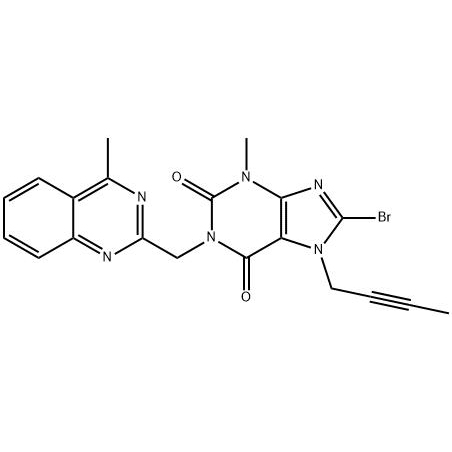
Short Lead Time for Reverse Osmosis System - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN
Short Lead Time for Reverse Osmosis System - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN
Short Lead Time for Reverse Osmosis System - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN Detail:
Use: Intermediate for Linagliptin.
Use:Intermediate for Linagliptin
Executive standard: enterprise standard
Assay:98-102%
Exterior:White to light yellow powder
Package: 25kg/drum
To analyze the existing linagliptin and its key intermediate 8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl -2-quinazolinyl)methyl)-1H-purine-2,6-dione (11) synthesis method, find a synthetic route suitable for industrial production. Method: summarize the different synthetic routes. Results and conclusions: Route 2.2 has a relatively simple process and lower cost, which is more suitable for industrial production.
8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione is a key intermediate in the synthesis of the hypoglycemic drug linagliptin. The synthesis of 1 uses methyl urea and cyanoacetic acid as starting materials, and undergoes six-step reactions of condensation, cyclization, nitrosation, reduction, cyclization, and bromine with a total yield of 46.3%. The structures of all intermediates were confirmed by 1HNMR.
The present invention relates to a simple preparation method of high-purity linagliptin. Quinazoline, the key intermediate for the one-pot preparation of linagliptin 8 bromo 7 (2 butyne 1 base) 3,7 dihydro 3 methyl 1 [(4 methyl 2 quinazolinyl) methyl] 1H Purine 2,6 dione, the intermediate is separated by filtration, and then reacted with (R)3 aminopiperidine dihydrochloride to obtain a solution containing linagliptin. After the solution containing linagliptin is processed again, Deliraliptin pure product. The preparation of the key intermediate of the present invention adopts a one-pot method, which is convenient to operate and improves the yield. After the key intermediate is separated, it is reacted with (R)3 aminopiperidine dihydrochloride, thereby Obtaining high-purity linagliptin also meets the production and declaration requirements of pharmaceutical companies to the greatest extent.
Product detail pictures:
Related Product Guide:
We've been convinced that with joint efforts, the enterprise between us will bring us mutual benefits. We could guarantee you item excellent and aggressive price tag for Short Lead Time for Reverse Osmosis System - 8-Bromo-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3 – JIN DUN , The product will supply to all over the world, such as: Leicester, Uzbekistan, Burundi, With strong technical strength and advanced production equipment, and SMS people purposefully , qualified, dedicated spirit of enterprise. Enterprises took the lead through the ISO 9001:2008 international quality management system certification, CE certification EU ; CCC.SGS.CQC other related product certification. We look forward to reactivating our company connection.
The goods we received and the sample sales staff display to us have the same quality, it is really a creditable manufacturer.



![OEM manufacturer Lotus Leaf Hydrophobic - 4-[4-[(5S)-5-(Aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-3-morpholinone Hydrochloride – JIN DUN](https://cdn.globalso.com/jindunchem-med/dc3948321.jpg)
![Reasonable price for Ozone Water Treatment System - 2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl] – JIN DUN](https://cdn.globalso.com/jindunchem-med/922e79ba.jpg)