6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl]
6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl]
This product is a light yellow film-coated tablet, white or almost white after removing the coating.
Indications
This product is suitable for the treatment of type 2 diabetes.
Monotherapy
This product is used in conjunction with diet control and exercise to improve blood sugar control in patients with type 2 diabetes.
Used in combination with metformin hydrochloride
When metformin hydrochloride alone cannot effectively control blood sugar, this product can be used in combination with metformin hydrochloride to improve blood sugar control in patients with type 2 diabetes on the basis of diet and exercise.
Used in combination with metformin hydrochloride and sulfonylureas.
When the combined use of metformin hydrochloride and sulfonylureas cannot effectively control blood sugar, this product can be used in combination with metformin hydrochloride and sulfonylureas to improve the blood sugar control of patients with type 2 diabetes on the basis of diet and exercise.
Medication restrictions
This product is not recommended for patients with type 1 diabetes or for the treatment of diabetic ketoacidosis.
Specification
(1) 10 mg; (2) 25 mg.
Dosage
Recommended dose.
The recommended dose of this product is 10 mg in the morning, once a day, on an empty stomach or after eating. In patients who tolerate this product, the dose can be increased to 25 mg (see [Clinical Trials]).
In patients with hypovolemia, it is recommended to correct the hypovolemia before starting to use this product (see [Precautions]).
Patients with renal impairment.
It is recommended to evaluate the renal function before starting to use this product, and then it should be evaluated regularly.
Patients whose eGFR is lower than 45 mL/min/1.73 m2 should not use this product.
Patients with eGFR higher than or equal to 45 mL/min/1.73 m2 do not need to adjust the dose.
If eGFR is continuously lower than 45 mL/min/1.73 m2, this product should be discontinued (see [Precautions]).
Patients with liver damage.
Patients with liver damage do not need to adjust the dose. Enpagliflozin exposure increased in patients with severe liver damage. The treatment experience of patients with severe liver damage is limited, therefore, it is not recommended for this part of the population.
Standard:enterprise standard
purity:≥99.0%
Exterior:white to off-white powder
Package: 25kg/drum

JIN DUN Medical has ISO qualification and meets GMP production standards, employed domestic and foreign drug synthesis experts with rich experience to guide company's R&D.




TECHNOL OGY ADVANTAGES
●High Pressure Catalytic Hydrogenation . High Pressure Hydrogenolysis Reaction . Cryogenic Reaction (<-78%C)
●Aromatic Heterocyclic Synthesis
●Rearrangement Reaction
●Chiral Resolution
●Heck, Suzuki,Negishi,Sonogashira . Gignard Reaction
Equipments
Our Lab has various experimental and testing equipments, such as: NMR (Bruker 400M)、HPLC、chiral-HPLC、LC-MS、LC-MS/MS (API 4000)、IR、UV、GC、GC-MS, Chromatography, Microwave Synthesizer, Parallel Synthesizer, Differential Scanning Calorimeter (DSC), Electron Microscope...
R&D Team
Jindun Medical has a group of professional R&D personnel, and employs many domestic and foreign drug synthesis experts to guide R&D, making our synthesis more accurate and efficient.

We have helped several top domestic pharmaceutical companies, such as Hansoh, Hengrui and HEC Pharm. Here we will show part of them.
Customzation Case One:
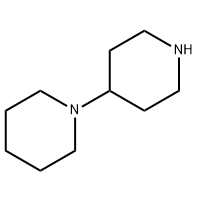
Cas No.: 110351-94-5
Customzation Case Two:
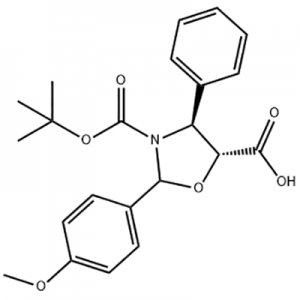
Cas No.: 144848-24-8
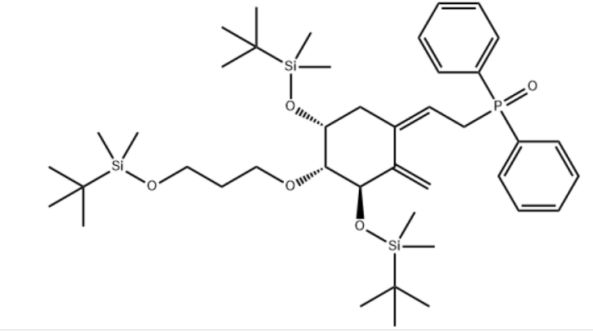
Customzation Case Three:
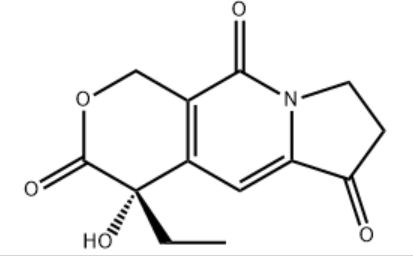
Cas No.: 200636-54-0
1.Customize New Intermediates or APIs. Same as above case sharing, customers have the demands for specific Intermediates or APIs, and they can not find the needed products in the market, then we can help to Customize.
2.Process Optimization for Old Products. Our team will help to Optimize and improve such production whose reaction route is old, production cost is high, and the efficiency is low. We can provide full documentation for technology transfer and process improvement, help customer for a more efficient production.
From drug targets to INDs, JIN DUN Medical provides you with one-stop personalized R&D solutions.
JIN DUN Medical insists on creating a team with dreams, making dignified products, meticulous, rigorous, and going all out to be a trusted partner and friend of customers!


![6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl] Featured Image](http://cdn.globalso.com/jindunchem-med/0ecf55f0.jpg)
![6-tetra-O-acteyl-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydrofu-ran-3-yl]oxy]phenyl]](http://cdn.globalso.com/jindunchem-med/0ecf55f0-300x300.jpg)


![1,4′-Bipiperidine]-1′-carbonyl chloride HCl](http://cdn.globalso.com/jindunchem-med/21.png)